|
Rassegna Stampa Scientifica Ottobre 2022
|
US: MMWR: NYTS: Teenagers Keep Vaping Despite Crackdowns
“High school students resumed taking the annual National Youth Tobacco Survey in school this year and 14 percent of them reported using e-cigarettes, underscoring how an upstart industry is dodging regulators’ efforts to spare a generation from nicotine addiction… One stark finding was that one in four of the high school students who were e-cigarette users reported vaping every day. Groups opposed to e-cigarettes and tobacco products were particularly troubled by one other result that reflected the highest frequency-of-use to date: Nearly half of the high school students who were vaping said they were doing so 20 to 30 days a month.” [Christina Jewett. Teenagers Keep Vaping Despite Crackdowns on E-Cigarettes, NY Times]
Teenagers Keep Vaping Despite Crackdowns on E-Cigarettes
While use among youths has fallen since the peaks of 2018-19, resumption of in-school classes this year shows students still have access to flavored, disposable vapes.
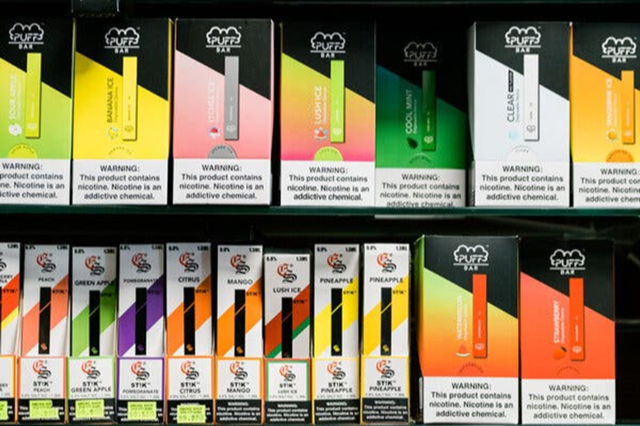
High school students reported strongly favoring fruit- and candy-flavored vapes.Credit...Gabriela Bhaskar for The New York Times
Oct. 6, 2022
High school students resumed taking the annual National Youth Tobacco Survey in school this year and 14 percent of them reported using e-cigarettes, underscoring how an upstart industry is dodging regulators’ efforts to spare a generation from nicotine addiction.
The number shows a slight change from 11 percent last year, but researchers cautioned against drawing comparisons to 2021’s survey, which was conducted differently because it took place when many schools were closed during the pandemic. The latest results were released by the Centers for Disease Control and Prevention on Thursday.
Though the age-old force of peer pressure may still be encouraging use, the percentage of high school students who reported vaping within the last 30 days was still far lower than record-high levels reached in 2019 of nearly 28 percent.
Overall, the survey found that 2.5 million middle and high school students, or about 9 percent, used e-cigarettes in the last 30 days. That puts their overall rate of use several times higher than that of adults, which is estimated at about 3 percent.
The survey, which was conducted from January through May of this year, showed that 85 percent of adolescent e-cigarette users favored vapes in fruit, dessert and candy flavors. Some mentioned PuffBar, Vuse and Juul as their favorite brand among those on the survey’s list.
But many said their favored e-cigarette brand was not one of the 13 listed. That finding highlights how nimble the industry has been in stamping an array of brand names on vapes with flavors like strawberry ice cream and fresh vanilla that are largely made in China and shipped from warehouses to corner stores and into e-commerce.
“What that shows is that playing Whac-A-Mole with a few products is not going to solve the problem,” said Vince Willmore, a spokesman for the Campaign for Tobacco-Free Kids. “As long as any flavored products are still on the market, kids are going to shift to them. To solve the problem, you have to clear the market of all flavored products.”
Read More on Smoking, Vaping and E-Cigarettes
- The Business of Addiction: McKinsey’s ties to opioid makers are well known, but for decades the consulting giant worked with Big Tobacco and has also advised Juul.
- Youth Vaping Settlement: Juul tentatively agreed to pay $438.5 millionto settle an investigation by nearly three dozen states over marketing and sales practices that they contend set off the teen vaping crisis.
- Ban Suspension: The Food and Drug Administration ordered Juul to stop selling e-cigarettes on the U.S. market, though it later suspended the banciting “scientific issues” that warrant a review of the decision.
- Nicotine Levels: The F.D.A. is planning to require tobacco companies to slash the amount of nicotine in their cigarettes. But experts warn that drastic cuts could prove disruptive and fuel underground markets.
One stark finding was that one in four of the high school students who were e-cigarette users reported vaping every day. Groups opposed to e-cigarettes and tobacco products were particularly troubled by one other result that reflected the highest frequency-of-use to date: Nearly half of the high school students who were vaping said they were doing so 20 to 30 days a month.
“That’s a real signal of addiction and setting up young people for a lifetime of addiction which they don’t want, they didn’t choose and they don’t like,” said Robin Koval, president of the Truth Initiative, a nonprofit organization aimed at eliminating youth tobacco use.
Linda Neff, chief of the epidemiology branch in the C.D.C.’s Office on Smoking and Health, said that the sheer number of young people continuing to vape suggested the agency needed to keep working to educate teens about the effects of nicotine addiction, which “harms the parts of the brain that control learning, mood and impulse control.”
“The frequency of use is disturbing,” she said. “It’s alarming.”
The Food and Drug Administration considers e-cigarettes to be generally beneficial to the extent that they provide an alternative to adult users of traditional cigarettes, which coat the lungs in tar. The agency’s hope for health gains, though, has existed in the shadow of a youth vaping crisis that exploded in 2018-19, prompting an outcry from parents, schools, lawmakers and public health experts.
The F.D.A. began to crack down on vape makers in 2019, banning many flavors and ordering manufacturers to apply for marketing authorization to keep their products on the market — an ongoing process. That effort has been challenged by e-cigarette makers who saw a loophole in making e-cigarettes with synthetic nicotine and jumped into the market with blueberry, kiwi and candy-flavored vapes.
This spring Congress gave the F.D.A. the authority to rein in those devices. The agency said it was reviewing about one million applications to sell synthetic nicotine products. In July, the agency gained authority to remove unauthorized non-tobacco products from the market but has said it needs to move methodically as it enforces the law.
On Thursday, the agency announced that it sent new warning letters to two companies that teenagers singled out as go-to brands in the survey. The F.D.A. issued its second warning to the maker of Puff Bar vapes, this time about its flavored synthetic nicotine products that the agency said were being sold illegally.
The F.D.A. also said Thursday that it denied marketing authorization to Hyde, a company that about 5 percent of adolescents wrote in on the survey as a favored brand — suggesting the rate is higher. The company’s website shows flavors including “pink burst” and “lemon drop.”
In a statement, the agency said that Hyde must stop selling its products or “risk enforcement action.”
Juul Labs, which had been widely blamed for fueling the teenage vaping crisis, pointed to the decline in popularity of its products among youths in a statement it released on Thursday. The company is awaiting a decision from the F.D.A. on its marketing application to remain on the market.
To some, the enforcement drive by the F.D.A. appears self-defeating. Amanda Wheeler, president of the American Vapor Manufacturers, said that the flood of denials faced by U.S. vape businesses were opening the door to foreign companies that would be more difficult to regulate.
As the agency’s rejections mount, “we will continue to see black market actors take advantage of F.D.A.’s wholesale destruction of the category,” Ms. Wheeler said.
The health consequences for teenagers who develop a nicotine addiction are just beginning to be understood. Dr. Rose Marie Robertson, science and medicine officer with the American Heart Association, said scientists were seeing toxic effects from the inhaled flavoring ingredients of e-cigarettes. She said researchers were also detecting signs of use on the heart and lungs.
“It took us 40 years to show that women would develop lung cancer more readily if they smoked,” Dr. Robertson said. “The fact that we’re seeing any effects at an early stage is very worrisome.”
The persistent rate of e-cigarette use among teenagers also concerns experts who were thrilled to see youth cigarette smoking rates fall steadily for years and remain in the single digits, with about 3.3 percent of middle and high school students reporting use in 2020.
“To have decades of progress wiped away by e-cigarettes has been astonishing to us who’ve been there all along,” Dr. Robertson said.
The full results of the survey, which will include levels of other tobacco product use, is expected out later this year.
Christina Jewett covers the Food and Drug Administration. She is an award-winning investigative journalist and has a strong interest in how the work of the F.D.A. affects the people who use regulated products. @By_Cjewett
https://www.nytimes.com/2022/10/06/health/teenage-vaping-e-cigarettes.html
Big Tobacco Heralds a Healthier World While Fighting Its Arrival
The industry continues to fight efforts to restrict certain products, like spending heavily to urge California voters to overturn a law banning tobacco flavors.
The fight shaping up over government restrictions on menthol and nicotine highlights the longstanding resistance of the tobacco industry to regulations, despite corporate claims of support for a smokeless alternative. [Photo Cutline]
By Julie Creswell and Matt Richtel
Nov. 6, 2022
For decades, public health advocates chipped away at the influence of Big Tobacco with measures aimed at discouraging cigarette use. But the bitter legal and political battles were just a prelude to the unfolding climactic clash that could determine the fate of smoking and whether these companies adapt or falter.
U.S. health officials have launched the most aggressive attack by far on cigarettes: Twin government proposals would ban menthol-flavored cigarettes and would limit nicotine levels to make traditional smoking less addictive. At the same time, the government is slowly embracing vaping as an alternative by authorizing the sale of some e-cigarettes, which can provide smokers a nicotine fix without many of the carcinogens.
The measures are the source of a clash expected to play out over the coming months and years in courtrooms, legislative hallways and regulatory hearings. For public health advocates, the steps are aimed at saving millions of lives and reducing the billions of dollars spent on smoking-related illnesses like cancer and heart disease.
Big Tobacco has said it embraces the transition — sort of.
“We have an unprecedented opportunity to move beyond smoking,” Billy Gifford, chief executive of Altria, one of the world’s biggest cigarette conglomerates and the parent company of Philip Morris USA, told Wall Street analysts and investors in late October. The opening slide of his presentation offered a company vision: “To responsibly lead the transition of adult smokers to a smoke-free future.”
Major cigarette companies, like Altria and R.J. Reynolds, acknowledge that cigarettes are dangerous and addictive, and they are heralding their investments in electronic cigarettes and other less-harmful alternatives to cigarettes. But, with much less fanfare, they are taking steps to slow the very smokeless future they claim to want: The companies have submitted letters protesting the proposed menthol ban in traditional cigarettes, and they have signaled they will similarly resist any efforts to lower nicotine levels.
And Big Tobacco isn’t just duking it out at the federal level, but fighting local initiatives. For example, in California, the industry has spent heavily to stop a 2020 law from taking effect that would ban the sale of flavored-tobacco products including menthol. Putting the law in place depends on a majority of state voters supporting a Nov. 8 ballot proposition favoring the law, and the industry has spent $22 million to to try to persuade voters to reject the measure and the flavor ban.
The California Coalition for Fairness, the tobacco industry-funded group behind the campaign that succeeded in getting the referendum on the ballot, argues the flavor ban “benefits the wealthy and special interests while costing jobs and cutting funding for education and health care.”
Mr. Gifford, in his late October call with investors, said of the flavor ban: We don’t believe science supports it.”
In various statements, R.J. Reynolds, owned by British American Tobacco and the second-largest cigarette company in the United States after Altria, has said it also embraces less harm but continues to hew to a business model that critics say puts public health second to profits.
In Reynolds’s filing against the menthol ban, it wrote that, broadly, it “fully supports F.D.A.’s goal of reducing tobacco-related disease.” But, it contended, “menthol smokers would simply switch to nonmenthol cigarettes or turn to riskier options such as illicit market cigarettes.” The company declined further comment beyond its filing.
As the smoking population in the United States has fallen to 13 percent from 21 percent in 2005, far from a peak of about 45 percent of adults in 1954, and public opinion has turned against cigarettes, the legal and political might of Big Tobacco has shrunk, too. A Gallup survey conducted in July found that 74 percent of Americans favored “requiring tobacco companies to lower nicotine levels in cigarettes to make them less addictive.” About 42 percent favored banning menthol-flavored cigarettes. (Under the current proposal, menthol e-cigarettes could be sold.)
But the industry still earns billions of dollars in revenues, and it hopes to use its remaining clout to stall these monumental proposals at the regulatory level and in court — or stop them altogether.
“This spring and summer, I would say, we’ve seen the most significant period of proposed regulations by the F.D.A. ever. Full stop,” said Sarah Milov, an associate professor of history at the University of Virginia and author of “The Cigarette: A Political History.” “With this industry, it’s all about where they are making their money. We will see them fight the menthol and nicotine rules, and that will be another demonstration of their continued commitment to combustible cigarettes.”
A broad group of allies has joined the tobacco industry in the fight against the menthol ban by. There are those with financial stakes in the outcome, like the National Association of Convenience Stores, which say they would lose billions of dollars in annual sales, and the New York City Newsstand Operators Association.
The menthol ban has also drawn opposition from think tanks like the Tax Foundation, which said federal and state governments could lose a combined $6.6 billion in tax revenues the first year. The American Civil Liberties Union has also opposed the ban, saying it would disproportionately affect communities of color.
Major cigarette companies, like Altria and R.J. Reynolds, submitted letters last summer protesting the proposed menthol ban in traditional cigarettes. [Photo Cutline]
In particular, the proposed ban has divided Black leaders across the country, especially since companies heavily marketed menthol cigarettes to Black smokers, who now prefer them at a much higher rate than white smokers do. While some welcomed the proposal as a way to lower cancer and heart disease, others expressed concerns that enforcing such a ban would lead to unwarranted police interactions with Black Americans. Big Tobacco has heavily lobbied against the ban with Black political leaders and retained some to help sew doubt and fear about the ban in communities around the country.
Many opponents have challenged the F.D.A.’s legal authority to regulate tobacco products in far-reaching ways. But no matter how the companies promote their position, industry critics say that their goal is to maintain the lucrative share of the cigarette market at all costs. No wonder: Sales in the U.S. totaled $65 billion in 2021 —- one-third of it from menthol — dwarfing sales of e-cigarettes.
“It’s absolutely false that they want to have their smoking customers quit or shift to less harmful tobacco products,” said Eric Lindblom, a senior scholar at the O’Neill Institute for National and Global Health Law at Georgetown University and a former adviser to the F.D.A. “If they were serious about having smokers quit, they would stop opposing any efforts at the federal, state and local level to regulate and tax smoking tobacco products more sharply.”
Traditional cigarettes have become more expensive, though. A study published this year in JAMA found that from 2015 to 2021, the number of packs of cigarettes sold in the United States fell to 9.1 billion a year from 12.5 billion, a 27 percent drop. To compensate, tobacco companies increased prices — rising 29.5 percent a pack during that period, to $7.22 from $5.57.
Inflation plays a role, too. In the first nine months of this year, Altria reported a steep 9 percent decline in sales volumes, with executives noting that customers were changing behaviors to save money, like buying single packs of cigarettes, rather than cartons.
Company share prices have also fallen.
“Most investors knew new regulation was coming, but the threat seemed far into the future,” said Christopher Growe, an analyst at financial services firm Stifel Financial. “I think menthol has more immediacy, but nicotine regulation is a long, long way away.”
The transformation of tobacco
On some level, the battle over menthol and nicotine limits extends the government’s efforts to chip away at smoking, even as the industry resists at every turn. But this moment is also fundamentally different. For the first time, many public health officials have embraced a strategy of harm reduction, which is not just to curb the cigarette market but to accept and even advocate for an alternative with e-cigarettes.
This strategy is not one that public health officials adopted lightly: For years, many were skeptical about legalizing e-cigarettes, worrying that the devices hooked a new generation on nicotine and lured young people into the vaping crisis.
Twin government proposals involve outlawing menthol-flavored cigarettes and limiting nicotine in cigarettes. At the same time, the government is slowly embracing an alternative by legalizing the sale of some e-cigarettes. [Photo Cutline]
While public health experts debated the merits of e-cigarettes, major companies argued that, absent that alternative or other products, there were no appealing options to help smokers quit.
Mitch Zeller, who retired this year from his post as director of the F.D.A.’s Center for Tobacco Products, said that for all his experience with the companies, he wasn’t sure that they would accept a smokeless future. How they respond to the new proposals will be “a test of their sincerity,” he said.
“It’s a day of reckoning for the industry,” Mr. Zeller said, adding of the tobacco companies. “They’ve got to make a decision.”
He acknowledged that the tobacco companies were in a tough position, having widely deployed “rhetoric” supporting alternatives but, at the same time, having to answer to shareholders whose returns were still reliant on cigarette sales and profits.
“They have a fiduciary duty to their shareholders,” he said. He added, however, that regulation might force the companies to adapt, no matter how hard they resisted.
Still, tobacco giants are pushing back against any efforts to curb sales. The industry has persistently sued to stop the federal government from requiring larger, graphic warnings on packages about the deadly risk of cigarettes. And Big Tobacco companies have continued to ply a tactic they’ve used for years: poaching former F.D.A. employees, mostly recently with Philip Morris International hiring Matt Holman, who was the chief of the science office in the agency’s Center For Tobacco Products.
The tobacco industry has been joined in the struggle against the menthol ban by broad group of allies, like the National Association of Convenience Stores and the NYC Newsstand Operators Association. [Photo Cutline]
If the F.D.A. pushes through a menthol ban, the tobacco industry will “dig in” and go to court, said Marc Scheineson, a former associate commissioner at the agency who is now a partner at the law firm Alston & Bird, which represents some smaller tobacco companies. “If there are rules that are put in place with the F.D.A. sort of ignoring valid scientific objections or criticisms, it will end up in court again.”
He noted a recent win for the Cigar Association of America, which challenged the F.D.A.’s regulation of premium cigars. In that case, U.S. District Judge Amit Mehta in Washington, D.C., said the F.D.A. had acted “arbitrarily and capriciously” and ignored or overlooked evidence provided by the industry. The case is still pending.
In another blow to the F.D.A., the U.S. Court of Appeals for the 11th Circuit in late August set aside marketing denial orders for six e-cigarette companies, saying the agency there, too, had been arbitrary and capricious in its decisions.
Mr. Scheineson said he hoped a compromise could be reached. He asked: Could nicotine be reduced in a slow, laddered way while allowing menthol cigarettes to be sold?
In the interim, all e-cigarette companies have had to apply to the F.D.A. to remain on the market, now that the agency has received expanded authority to regulate vaping devices and e-cigarettes. The F.D.A. is wading through applications for 350 products, according to a letter published in Augustby Brian King, the director for the Center for Tobacco Products. In the last two years, the agency has authorized the sale of about two dozen vaping products.
And the biggest tobacco companies are vying for their piece of the budding market. Last year, the F.D.A. approved several Vuse products by Reynolds. However, the agency has not yet ruled on the sale of Vuse Alto, the company’s biggest seller to date, which accounted for 95 percent of its e-cigarette sales last year and displaced Juul as the top-selling vaping product. Vuse Alto has gained in popularity in recent years for its small, sleek design, longer battery life and the fact that it wasn’t mired in the same teenage-use controversy as Juul.
Altria’s strategy had long appeared to be pinned to its relationship with Juul Labs. In 2018, Altria paid $12.8 billion for a 35 percent stake in Juul. But even before Juul lost its initial bid in June for authorization to keep selling certain products on the U.S. market, the company’s products had been severely restricted by public pressure to pull flavored e-pods off the market out of concerns for their appeal to teenagers. The F.D.A. reversed itself this summer and is granting an additional review to Juul’s application for certain tobacco and menthol products to stay on the market.
By late September, Altria had taken a more than $12 billion cumulative loss on Juul, valuing the investment at $350 million. Altria said it ended its noncompete agreement with Juul, opening up the possibility it could acquire another e-cigarette company to compete in the space, some analysts predict. Meanwhile, reports emerged in October that Juul might seek bankruptcy protection.
Besides Juul, Altria also has stakes in companies that make nicotine pouches, a product that is placed between the cheek and jaw.
Another category of cigarette alternatives are known as “heat-not-burn tobacco sticks.” In October, Altria announced that it sold the U.S. rights to sell IQOS, a heat-not-burn tobacco stick, for $2.7 billion to Philip Morris International.
To fill the void, Altria promptly announced a new joint venture with Japan Tobacco to develop a heat-not-burn stick called Ploom for the U.S. market.
On Altria’s call with investors in late October, Mr. Growe, the Wall Street analyst from Stifel, asked the company’s chief executive when a new Ploom product might be available. “Do you have a reasonable time frame for launching a product in the U.S.” he asked, and then added a few sentences later: “Or am I getting ahead of myself here?”
“I think you’re getting ahead of yourself a little bit,” said Mr. Gifford, Altria’s chief executive.
“Maybe underlying your question is: ‘why are you taking so long,” Mr. Gifford continued. “And I think it goes back to, look, we want to be disciplined.”
Mr. Gifford said that Altria absolutely wants to create an alternative to the cigarette, but not in a hasty fashion. “We need to go about it in a thoughtful manner.”
Julie Creswell is a New York-based reporter. She has covered banks, private equity, retail and health care. She previously worked for Fortune Magazine and also wrote about debt, monetary policy and mutual funds at Dow Jones. @julie_creswell
Matt Richtel is a best-selling author and Pulitzer Prize-winning reporter based in San Francisco. He joined The Times in 2000, and his work has focused on science, technology, business and narrative-driven storytelling around these issues. @mrichtel
https://www.nytimes.com/2022/11/06/health/tobacco-fda-menthol-ban-nicotine.html






















